Real World Evidence Courses
Real World Evidence Courses - A practical taxonomy of observational research. Understand real world evidence (rwe) and learn how to use it. This program is set at a beginner or novice level. Participants will learn through expert video’s, blogs, interviews and individual assignments. Identifying who within the life sciences industry uses and engages with rwe? During this online course, students will: Understanding the methodology behind complex statistical methods used for rwe in published scientific papers. Understand how rwe can be used for decision making. George hripcsak, patrick ryan, anna ostropolets and karthik natarajan will serve as faculty for this session, which will be limited to 30. Learn how rwd is collected from various healthcare settings. This program is set at a beginner or novice level. Participants will have the opportunity to learn from key experts in the field. Learn how rwd is collected from various healthcare settings. Participants will learn through expert video’s, blogs, interviews and individual assignments. It addresses the limitations of traditional clinical trials and highlights rwe's advantages. Understanding the methodology behind complex statistical methods used for rwe in published scientific papers. Rwd refers to data collected from patients' health status and routine healthcare delivery, while rwe represents clinical insights derived from analyzing rwd. It will help you overcome obstacles and perfectly match your study and project objectives. Develop their knowledge of concepts, study designs, and methods in pharmacoepidemiology; Dive into the world of health economics and outcomes research (heor) through our gpat tutorials on the applications of randomized controlled trials. Introduction to real world evidence. Identifying who within the life sciences industry uses and engages with rwe? Participants will have the opportunity to learn from key experts in the field. As part of the reauth. Rwe strategy and study designs. Identifying who within the life sciences industry uses and engages with rwe? Rwd refers to data collected from patients' health status and routine healthcare delivery, while rwe represents clinical insights derived from analyzing rwd. Rwe strategy and study designs. Understanding the methodology behind complex statistical methods used for rwe in published scientific papers. Dive into the world of health economics. You’ll learn how to generate evidence to change medicine and improve people’s lives. Dive into the world of health economics and outcomes research (heor) through our gpat tutorials on the applications of randomized controlled trials. Rwe strategy and study designs. Rwe strategy and study designs. Understand real world evidence (rwe) and learn how to use it. This program is set at a beginner or novice level. Identifying who within the life sciences industry uses and engages with rwe? Rwe strategy and study designs. Recognizing how rwe fits within the health industry ecosystem? Understand real world evidence (rwe) and learn how to use it. Participants will learn through expert video’s, blogs, interviews and individual assignments. Understand how rwe can be used for decision making. During this online course, students will: Exploring the power of real world evidence (rwe) in pharma. Understanding the methodology behind complex statistical methods used for rwe in published scientific papers. Understanding the methodology behind complex statistical methods used for rwe in published scientific papers. During this online course, students will: Understanding the methodology behind complex statistical methods used for rwe in published scientific papers. Rwd refers to data collected from patients' health status and routine healthcare delivery, while rwe represents clinical insights derived from analyzing rwd. You’ll learn how to. Understanding the methodology behind complex statistical methods used for rwe in published scientific papers. Understanding the methodology behind complex statistical methods used for rwe in published scientific papers. During this online course, students will: It addresses the limitations of traditional clinical trials and highlights rwe's advantages. Exploring the power of real world evidence (rwe) in pharma. As part of the reauth. Understanding the methodology behind complex statistical methods used for rwe in published scientific papers. Participants will learn through expert video’s, blogs, interviews and individual assignments. This program is set at a beginner or novice level. Dive into the world of health economics and outcomes research (heor) through our gpat tutorials on the applications of randomized. Understanding the methodology behind complex statistical methods used for rwe in published scientific papers. On this course you will learn how real world evidence can be used in healthcare, exploring current trends and existing methodologies for using it. George hripcsak, patrick ryan, anna ostropolets and karthik natarajan will serve as faculty for this session, which will be limited to 30.. Real world data (rwd) is the huge quantity of data that falls outside the boundaries of controlled clinical trials, data that During this online course, students will: This program is set at a beginner or novice level. You’ll learn how to generate evidence to change medicine and improve people’s lives. Learn how rwd is collected from various healthcare settings. Introduction to real world evidence. It addresses the limitations of traditional clinical trials and highlights rwe's advantages. Dive into the world of health economics and outcomes research (heor) through our gpat tutorials on the applications of randomized controlled trials. Understand how rwe can be used for decision making. Participants will have the opportunity to learn from key experts in the field. Participants will learn through expert video’s, blogs, interviews and individual assignments. On this course you will learn how real world evidence can be used in healthcare, exploring current trends and existing methodologies for using it. Rwe strategy and study designs. Rwe strategy and study designs. You will consider ethics, design thinking, commercial applications, and the limitations of rwe. As part of the reauth. A practical taxonomy of observational research. During this online course, students will: Understanding the methodology behind complex statistical methods used for rwe in published scientific papers. Identifying who within the life sciences industry uses and engages with rwe? A practical taxonomy of observational research.RealWorld Evidence National Health Council
IQVIA Canada on LinkedIn Real World Evidence Essentials Course
Real World Evidence Conference
RealWorld Evidence Encyclopedia MDPI
An Introduction to RealWorld Data & RealWorld Evidence A Virtual
AS Training Programs AS
Using realworld evidence to supplement randomized controlled trials
Real World Evidence RWE Training Royed Training
Leveraging Real World Evidence in Regulatory Submissions of Medical
9 Ways RealWorld Evidence is Changing Healthcare // ArborMetrix
Develop Their Knowledge Of Concepts, Study Designs, And Methods In Pharmacoepidemiology;
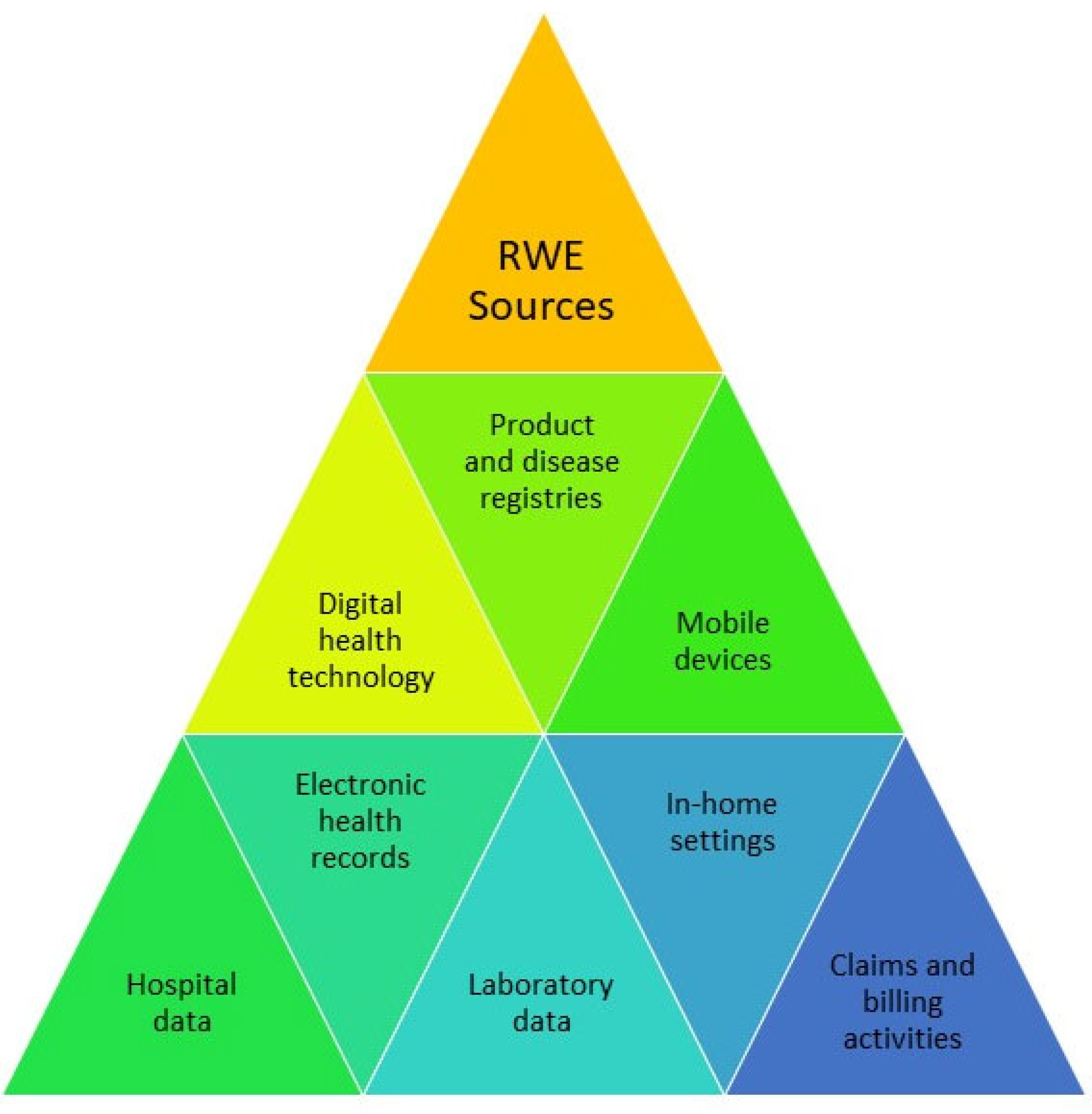
Real World Data (Rwd) Is The Huge Quantity Of Data That Falls Outside The Boundaries Of Controlled Clinical Trials, Data That
Understanding The Methodology Behind Complex Statistical Methods Used For Rwe In Published Scientific Papers.
Rwd Refers To Data Collected From Patients' Health Status And Routine Healthcare Delivery, While Rwe Represents Clinical Insights Derived From Analyzing Rwd.
Related Post: